Page 41 - MBCA_FULL REPORT_FINAL_FOR_WEB
P. 41
We then reviewed all targeted therapy trials and found 118 new drugs, vaccines, or new
combinations of drugs being tested. Appendix 3 lists the drug, or combination of drugs (if
applicable), molecular targets, and biomarkers/cancer subtype being tested in these clinical
trials according to the hallmarks of cancer categories.
TNBC Trials
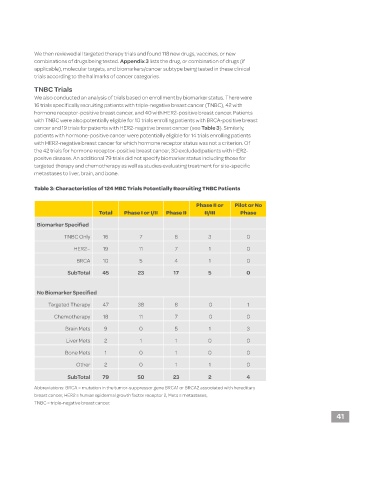
We also conducted an analysis of trials based on enrollment by biomarker status. There were
16 trials specifically recruiting patients with triple-negative breast cancer (TNBC), 42 with
hormone receptor-positive breast cancer, and 40 with HER2-positive breast cancer. Patients
with TNBC were also potentially eligible for 10 trials enrolling patients with BRCA-positive breast
cancer and 19 trials for patients with HER2-negative breast cancer (see Table 3). Similarly,
patients with hormone-positive cancer were potentially eligible for 14 trials enrolling patients
with HER2-negative breast cancer for which hormone receptor status was not a criterion. Of
the 42 trials for hormone receptor-positive breast cancer, 30 excluded patients with HER2-
positve disease. An additional 79 trials did not specify biomarker status including those for
targeted therapy and chemotherapy as well as studies evaluating treatment for site-specific
metastases to liver, brain, and bone.
Table 3: Characteristics of 124 MBC Trials Potentially Recruiting TNBC Patients
Phase II or Pilot or No
Total Phase I or I/II Phase II II/III Phase
Biomarker Specified
TNBC Only 16 7 6 3 0
HER2− 19 11 7 1 0
BRCA 10 5 4 1 0
SubTotal 45 23 17 5 0
No Biomarker Specified
Targeted Therapy 47 38 8 0 1
Chemotherapy 18 11 7 0 0
Brain Mets 9 0 5 1 3
Liver Mets 2 1 1 0 0
Bone Mets 1 0 1 0 0
Other 2 0 1 1 0
SubTotal 79 50 23 2 4
Abbreviations: BRCA = mutation in the tumor-suppressor gene BRCA1 or BRCA2 associated with hereditary
breast cancer, HER2 = human epidermal growth factor receptor 2, Mets = metastases,
TNBC = triple-negative breast cancer.
41